Cutaneous Leishmaniasis
P.K. Ghatak, M.D.
Oriental sore is present in a wide area of the world. The majority of the cases are from the New World countries - Brazil, Bolivia, Peru, Panama, and countries around the Mediterranean Sea. CL (Cutaneous Leishmaniasis) is also prevalent in the Old World, the Middle East and Central Africa.
CL is a parasitic disease of the skin and the mucous membranes of the nose, mouth and throat. The parasite is Leishmania, which belongs to the Trypanosomatidae family and the Kinetoplastids order. CT is transmitted to humans by Sandflies.
Incidence of CL.
About 600,000 to 1 million new cases of CL occur each year. The mortality is few and comes from secondary bacterial or fungal infections, but the morbidity is a serious problem.
Anatomy of Leishmania.
Leishmania is a unicellular organism that exists in two different anatomical forms. In the insect vector, Leishmania is an oblong, flagellated form called Promastigotes, which reside in the proboscis of the sandfly. A small, round, non-flagellated form called Amastigote is ingested by the sandfly from humans during feeding. In the gut of the sandfly, the parasites change to flagellated form.
The nucleus of Leishmania is present in the center of the cell; in front of the nucleus in the cytoplasm, a mass of mitochondrial DNA is present called the Kinetoplast. From the Kinetoplast a flagellum originates and extends outside. In the cytoplasm, a single strand of mitochondrion and Golgi apparatus are also present.
Leishmania have a unique DNA and glycosomes (peroxisome-like organelles). It was believed that Leishmania did not propagate by sexual union, but recent studies provided contrary evidence that amastigotes exchange nuclear material during binary fission. Leishmania has 34 to 36 chromosomes and during cell division, the chromosomes do not condense.
Life Cycle of Leishmania.
The flagellated Promastigotes infect humans. Sandfly bites introduce the Promastigotes to the human victims. The macrophages, neutrophils and phagocytes engulf the parasite but are unable to break down its surface membrane. Inside the cells, the Promastigotes change into Amastigotes. In the macrophages, the amastigotes multiply rapidly to such a number that the cell bursts open and the newly released amastigotes infect other tissues.
Inside the sandfly.
The sandfly takes a blood meal and swallows macrophages loaded with amastigotes, inside the gut, the macrophage wall breaks down, releasing the amastigotes. Amastigotes transform into Promastigotes and divides and move to proboscis of the sandfly.
20 different species of Leishmania produce CL., and 90 different species of sandflies are the vectors. The important Leishmania species in the Old World are L. infantum, L. donovani, L. major, L. aethiopia. In the New world's species are L.mexicana, L.infuntum, L.guyanensis, L.amazonensis, L.braziliensis, and L.panamanensis.
The Phlebotomy sandfly is the vector in the old world and Lutzomyia sandfly in the new world. A species of sandfly harbors only certain Leishmania species and that limits one type of CL lesion confined to a local area and absent from the adjoining village or locality.
Development of the Skin lesions and Clinical Types.
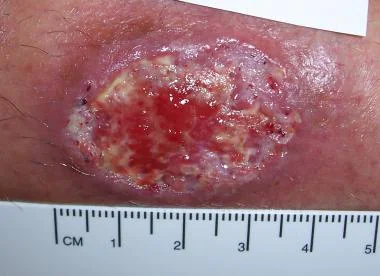
The sandfly bites on the face, forearms, hands and legs. The bites are painful. A red papule develops at the bite site, which turns into a nodule. Several days or weeks later, the nodule ulcerates. This is the beginning of the chronic indolent Oriental sore.
The subsequent healing or progression of CL depends on (a) Leishmania species.(b) victims' immune status - both the innate and acquired immunity. (c) Intercurrent immune diseases. (d) suppressed immunity by drugs, which lowers cellular immune functions or delays and diminishes immune globulin synthesis.
Clinical types of CL. (a) Limited Cutaneous Leishmaniasis.(b) Recidivans Leishmaniasis.(c) Mucocutaneous Leishmaniasis. (d) Anergic Diffuse Dermal Leishmaniasis. (e) Post Kala-azar Dermal Leishmaniasis.
Clinical features.
(a) Limited Cutaneous Leishmania. The initial lesion does not progress to ulcers because the victims have good levels of immunity. The lesions heal spontaneously with scars, and patients develop permanent immunity.
(b) Recidivans Leishmaniasis. The initial lesion heals with scars, and months or years may pass without further disease activities. Then new skin lesions begin to appear at or near the scars. The process goes on for a while, and ultimately the skin lesions heal. Generally, no medication is required.
(c) Mucocutaneous Leishmaniasis. It is the most devastating form of CL. The initial lesions develop around the nostrils, mouth, and eyes. Satellite lesions may develop in the throat. The mucosal lesions produce tissue necrosis and usually penetrate into the deeper layers of tissues and erode the muscles, cartilages and bones. Without medical therapy, no recovery is possible. The WHO recommends that all Mucocutaneous Leishmaniasis should be treated and the WHO provides guidance and medications in participating countries.
(d) Anergic Diffuse Dermal Leishmaniasis. The patients are immune deficient and usually have HIV infection. The initial lesions are papules and nodules, but lesions do ulcerate. Because of the lack of immune cellular response, multiple lesions appear, usually on the face, arms and buttocks. Later, the entire body is covered by several forms of skin lesions – plaques, papules and nodules. The appearance of the patient resembles nodular leprosy, however, no nerve damage occurs in this form of leishmaniasis. Treatment with medications is not very effective and where initial success is achieved, the recurrence of new lesions is common.
(e). Post Kala-azar Dermal Leishmaniasis. Visceral leishmaniasis is better known as Kala-azar. 2 to 20 years after the cure of Kala-azar, some patients develop hypopigmented macules, nodules, plaques and erythematous plaques on the face and other areas of the body. The lesions are recurrent and may persist for years.
Diagnosis of CL.
Biopsy. The demonstration of amastigotes within the macrophages is the standard diagnostic method. If there is no blood in the smear and stained with Geismar or Wright stains, a trained person can identify amastigotes without difficulty. An alternative to a biopsy is the aspiration of clear serum from the lesions. This method is equally sensitive. However, in certain forms of CL, the parasite numbers are few. So more and more reliance is shifting towards antibody detection by ELISA or antigen DNA detection by PCR test.
Treatment of CL.
The drug treatment is standardized by the WHO and the CDC in the USA. Few cases of CL were detected in the returning veterans from Afghanistan and Syria. In addition to standard therapy, each country has a host of local treatment protocols which are not effective but popular among the locals. Some of them are Cryotherapy, Heat therapy at 40 to 42 degrees C, Paromomycin topical preparations, infiltration of the ulcers with the solution of Sodium Stibogluconate, and urea 10 -15 % solutions. Oral dapsone, Oral Allopurinol and Oral antifungal agents
Standard therapy.
Pentavalent Antimony agents.
Two commercial preparations are available -Sodium stibogluconate (pentostam) and pentostam eglumine Antimonate (Glucantime). Both of these are given intravenously for 10 to 20 days
Liposomal amphotericin B. It is an antifungal drug and is also effective in leishmaniasis but expensive.
Oral Pentostam, pentostam
Oral Miltefosine. It is a wide spectrum antibiotic also effective against Leishmania. Initially, it was approved for cancer therapy.
Various combinations of drug therapy are practiced based on the leishmania species and the degree of tissue damage.
Prognosis.
Mucocutaneous and Anergic diffuse dermal leishmaniasis have poorer prognosis because of destructive facial lesions and disfigurements. Mortality is negligible as such, but secondary infection may lead to septicemia and deaths.
Vaccine: No commercial vaccine is produced for Leishmania disease. Russia made a vaccine using attenuated L. major and used in Russian endemic areas with 50 % success in controlling new skin lesions, but the vaccine does not protect against infection from other species than L. major, and also has some troublesome side effects.
*************************************************