Blood flukes
Scistosoma hematobium
Snail fever
P.K. Ghatak, M.D
Blood Fluke infection is the 3rd flatworm (Trematode) parasitic disease of humans discussed here, Liver fluke and Lung fluke were the other two mentioned in the earlier blogs.
Schistosoma hematobium is the parasite and the disease produced by the worm is called Schistosomiasis, also known as Bilharziasis and Snail fever. Schistosoma means a split - body. The shorter and stouter adult male has a longitudinal cleft along the length of the body and in that space, a slender, longer female worm is held in embrace by the male for its life. A German doctor, Theodor Maximilian Bilharzia, while serving in Egypt, discovered the flatworm parasite in 1851, and the disease is named after him.
Two distinct illnesses are produced by Schistosoma species.
Urogenital Schistosomiasis. Produced by S. hematobium. The worms reside in the venules (small veins) of the urogenital organs.
Gastrointestinal & Biliary Schistosomiasis. Several Schistosoma species are responsible and they are named according to the initial case originating country. The parasites invade the venules of the GI and biliary systems.
The life cycles of all the species of Schistosoma are identical and closely resemble those of the other trematodes.
Humans are the final host and act as the reservoir of the parasite. Other primates, ruminant farm animals and rodents are additional reservoirs.
Incidence of Bilharziasis.
The WHO estimates 200 million active cases are seen annually. Genitourinary bilharziasis is common in the Nile River Valley of Egypt and the adjoining northern African countries. Gastrointestinal/biliary bilharziasis occurs in China and countries surrounding the South China Sea. It is also prevalent in South America and the Caribbean islands. Indigenous bilharziasis does not happen in the USA.
Life Cycle of Schistosoma.
Human and animal feces and urine contaminate rivers, lakes and other bodies of water. Digging canals for irrigation has spread the risk of bilharziasis in much wider areas. Inundation during the rainy season is another hazard.
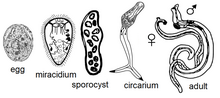
Schistosoma development stages are - Egg, Miracidia, Sporocyst, Cercaria and Adult.
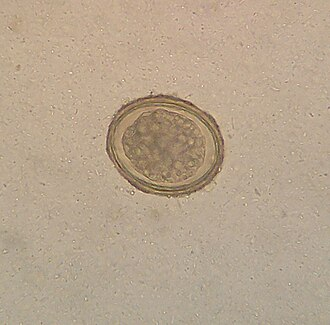
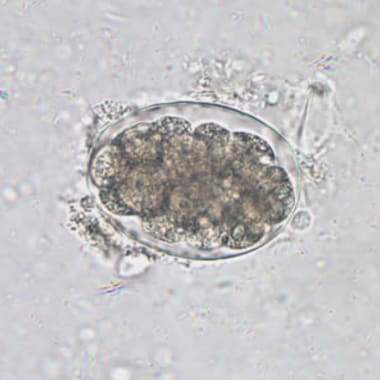
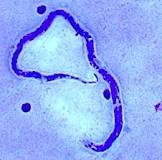
Eggs: An embryonated egg has a spine, and the position of the spine distinguishes one species from the other. Each egg is elongated or oval, measuring about 175 X 45 micrometers. Inside an egg, one embryo is in the development stage. A hinged door at the head-end opens and lets a grown larva out in the water.
Embryos: Miracidia larva is 200 micrometers long and covered with cilia and is a free swimmer. Miracidia larva lives in the water only a few hours and must find the proper snails that live in sweet water to multiply and develop further into infective larvae.
Sporocytes: Miracidia after entering inside the snail, move to softer tissue and transform into cysts. Cyst develops many daughter cysts and the daughter cysts move to newer locations and continue to develop into Cercaria larvae.
Cercaria larva is 500 micrometers long, has a tapering head and a forked tail. Cercariae live only 3 days. It takes only a few minutes for cercariae to enter into the body of their victims by penetrating the skin. It drops its tail and moves inside the veins. Inside the blood vessels, it becomes a round ball and is carried by the blood to the heart, lungs, and finally to the liver. In the liver, cercariae develop in about 3 weeks into adult male and female worms.
Adult worms: Adult worms are 7 to 29 mm long, have a cylindrical body, colored gray-white, have two suckers and an alimentary canal but no anus and the body is filled with reproductive organs.
Subsequently, the life of Schistosoma depends on the species and victims.
|
Schistosoma species |
Definitive host |
Site of infection |
Eggs discharged in |
Endemic area |
|
S. hematobium |
Humans, other primates |
Genitourinary system |
Urine |
Africa |
|
S. japonicum |
Humans, carnivores, ruminants | GI & biliary mesenteric veins |
feces |
South-East Asia |
S. mansoni |
Humans, rodents |
As above |
feces |
Africa |
|
S.mekongi |
Humans, dogs |
As above |
feces |
South-East Asia |
|
S. intercalatum |
Humans, rodents and cattle | As above |
feces |
South-East Asia |
Symptoms: The initial infection produces no symptoms. Some people develop an itch at the skin penetration sites and it is called swimmers' itch. Some others develop eosinophilia and patchy pneumonia.
Acute symptoms. Eggs produce allergic reactions known as Katayama fever. It manifests as fever, weakness, fatigue, and lymphadenitis.
Abdominal pain, low grade fever and eosinophilia develop in others.
Chronic symptoms. Many eggs are carried away to different organs. The eggs get embedded in the tissues and generate immune reactions. The initial inflammatory reaction is followed by tissue necrosis, fibrosis and granuloma formation. Small granulomas coalesce together into polyps. Polyps are seen in the urinary bladder and esophagus, stomach and intestine.
Genitourinary Bilharziasis: Hematurrhea, painful urination, urinary tract infection, glomerulonephritis, deformed external genitalia, calcified lesions surrounding the embedded eggs in tissues, specially in the urinary bladder, are common. Carcinoma of the bladder also develops. Fibrosis of the various components of the reproductive organs leads to difficulty in pregnancy and miscarriage. Blood in semen in a male patient is a striking feature.
Gastrointestinal and biliary bilharziasis:
Abdominal pain and diarrhea, dysphagia, bleeding varies in the esophagus and stomach producing anemia and malnutrition. Liver and spleen enlargement, anemia and leukocytopenia develop due to hypersplenism. Calcifications of blood vessels lead to various ischemic symptoms.
Distal organ involvement:
CNS. In the brain, the eggs are calcified and cause seizures, headaches and paralysis of limbs.
In the spinal cord: Transverse myelitis is a serious problem
Lungs: In the chronic stage, Pulmonary artery stenosis and calcifications produce pulmonary hypertension.
Heart: Myocarditis and heart failure occur.
Diagnosis: Stool examination detects eggs and the shape of eggs and the characteristics of the spine help diagnose the Schistosoma species.
Egg characteristics:
S.hematobium – Eggs are oval-shaped, the spine is long and sharp and attached to the terminal end.
S. japonicum - Eggs are round. The spine is rudimentary and appears like a hook and is attached to the lateral side.
S. mansoni –Eggs are elongated and the spine is attached at the posterior end of a side.
S. mecongi – Eggs are 30–45 micrometers long and have a short lateral spine.
S. intercalatum – Eggs are oval-shaped and the spine is attached to the terminal end; Its eggs resemble S. hematobium eggs.
In S. intercalatum infection, few eggs are excreted in the feces. The infection is in the lower colon and rectum. A biopsy of the rectum is required.
In CNS infection, more reliable tests are ELISA antibody test and parasite DNA identification by PCR test.
Treatment: Praziquantel is a very effective drug in killing adult worms but immature worms are not killed. A person is usually infected repeatedly, and both mature and immature adult worms are present at the same time. So Praziquantel is repeated weeks later. The Cure rate is 90 %.
Special features of Schistosoma. The adult worm does not produce inflammation or allergic reactions, only the eggs are allergenic.
The adult worm is classified as a flatworm, but it resembles round worms. The worms, unlike other flukes, are not hermaphrodites, the male and female sexes are separate.
****************************
.
in other primates, ruminants, rodents and cattle.
Adult The adult worms are 1–2 cm long with a cylindrical body that features two terminal suckers, a complex tegument, a blind digestive tract, and reproductive organs Schistosoma spp. [these species cause schistosomiasis/bilharzia in humans and ruminants]
Parasite morphology: Blood flukes form five different developmental stages: eggs, miracidia, sporocysts, cercariae and adult worms. Eggs are round to oval in shape, operculate (hinged at one end) and contain a developing embryonic larva (miracidium). Differences in egg morphology can be used to distinguish between Schistosoma species: S. mansoni producing oval eggs (115-175 x 45-7µm) with a sharp lateral spine, S. japonicum forming round eggs (70-100 x 50-70µm) with a rudimentary lateral spine; and S. haematobium producing oval eggs (110-170 x 40-70µm) with a sharp terminal spine. Miracidia are elliptical free-swimming larval stages (~200µm long) covered with cilia. Sporocysts appear as pleomorphic sac-like bodies which contain developing cercariae. Mature cercariae are elongate free-swimming larval stages (400-600µm long) consisting of a tapering head (with prominent penetration glands) and a forked tail (furcocercous). Adult flukes are elongate tubular worms (10-20mm long), with rudimentary oral and ventral suckers. Males are shorter and stouter than females, and they have a longitudinal cleft (gynecophoral canal or schist) in which the longer slender female lies folded.
Theodor Maximilian Bilharz
Regarding Katayama syndrome (a possible clinical manifestation of schistosomiasis in naive patients characterized by fever, cough, myalgia, headache and abdominal tenderness [19]), descriptions fitting its clinical manifestations can be found in ancient books of traditional Chinese medicine referring to more than 2400 years ago